Protein-Protein Interactions: The Building Blocks of Cellular Machinery
Proteins are the key molecules responsible for virtually every process in the cell, and many of their functions depend on interactions with other proteins. Protein-protein interactions (PPIs) form the fundamental basis of cellular networks, creating the building blocks of cellular machinery. These interactions are essential for processes such as signal transduction, metabolic regulation, and the structural integrity of cells. Understanding the mechanisms and dynamics of PPIs is crucial to decoding how cells function and respond to their environment.
In this blog, we’ll explore the different types of protein-protein interactions, their structural features, the methods used to study them, and their significance in biological processes. This technical dive will also highlight how disruptions in PPIs contribute to disease, making them a key focus for therapeutic interventions.
1. What Are Protein-Protein Interactions (PPIs)?
Protein-protein interactions occur when two or more proteins bind together, often transiently, to carry out a biological function. These interactions can vary from weak, reversible associations to strong, stable complexes. Proteins rarely function in isolation; instead, they form complexes with other proteins to participate in larger cellular processes such as enzyme cascades, transcriptional regulation, and signal transduction pathways.
PPIs can be classified based on various factors, including their duration (transient vs. stable), the number of proteins involved (binary vs. multimeric), and their structural basis (domain-domain, domain-peptide, or domain-motif interactions).
2. Types of Protein-Protein Interactions
A. Transient vs. Stable Interactions
- Transient Interactions: These interactions are typically short-lived and reversible. Transient interactions are crucial for signaling pathways, where proteins need to interact briefly to transmit signals across the cell. These interactions often occur in response to specific stimuli, such as hormonal signals or changes in cellular conditions (e.g., phosphorylation).
- Example: The interaction between kinases and their substrates is typically transient. Kinases bind to specific proteins, phosphorylate them, and then dissociate after the modification is complete.
- Stable Interactions: In contrast, stable interactions involve the formation of long-lasting protein complexes. These complexes often serve structural or enzymatic roles, with the proteins remaining associated throughout their functional lifetime.
- Example: Hemoglobin, a tetrameric complex composed of two alpha and two beta subunits, is a stable complex that persists to transport oxygen in the blood.
B. Homomeric vs. Heteromeric Interactions
- Homomeric Interactions: These interactions occur between identical protein molecules. Homomeric proteins often form oligomeric structures such as dimers, trimers, or higher-order assemblies. Homomeric interactions are common in structural proteins, enzymes, and transporters.
- Example: The enzyme aspartate transcarbamoylase forms a homotrimeric complex, with each subunit contributing to the overall catalytic function.
- Heteromeric Interactions: In contrast, heteromeric interactions involve two or more different proteins. Heteromeric complexes are essential for most biological functions, from signaling cascades to the formation of multi-enzyme complexes.
- Example: The ribosome, a complex machine responsible for protein synthesis, is composed of both ribosomal proteins and RNA. Its function depends on the coordinated interactions between these components.
C. Domain-Domain and Domain-Peptide Interactions
Proteins consist of structural and functional units called domains, which can mediate interactions with other proteins. The nature of these interactions varies depending on the structure and function of the domains involved:
- Domain-Domain Interactions: These interactions occur between two folded protein domains. Domain-domain interactions often result in the formation of large, stable complexes.
- Example: The SH2 domain of proteins like Grb2 specifically recognizes phosphorylated tyrosine residues on other proteins, allowing for the assembly of signaling complexes in response to growth factor stimulation.
- Domain-Peptide Interactions: Some domains, such as SH3 domains, recognize short linear motifs (peptides) on other proteins. These interactions are typically transient but play essential roles in cellular signaling pathways.
- Example: SH3 domains bind to proline-rich motifs in proteins involved in the regulation of the cytoskeleton, such as those found in the adapter protein Crk.
3. Structural Basis of Protein-Protein Interactions
The stability and specificity of PPIs depend on the structural complementarity between the interacting surfaces of the proteins involved. These surfaces are typically large, hydrophobic patches that exclude water and allow for tight packing of amino acid residues. However, electrostatic interactions, hydrogen bonds, and van der Waals forces also contribute to the stability of the interaction.
- Hydrophobic Interactions: The majority of PPIs are mediated by hydrophobic interactions, where non-polar amino acid side chains (such as leucine, isoleucine, and phenylalanine) pack together in the interior of the complex, away from water.
- Example: The dimerization of transcription factors like the basic leucine zipper (bZIP) proteins is driven by hydrophobic interactions between the leucine residues in their coiled-coil domains.
- Hydrogen Bonds and Electrostatic Interactions: In addition to hydrophobic forces, hydrogen bonds (between side chains like serine, threonine, or asparagine) and electrostatic interactions (between charged residues like lysine and glutamate) contribute to the specificity of protein-protein binding.
- Example: In antibody-antigen interactions, hydrogen bonds and electrostatic interactions allow the antigen-binding site of the antibody to specifically recognize and bind its antigen with high affinity.
4. Biological Significance of Protein-Protein Interactions
PPIs are integral to virtually all cellular functions. They form the core of multiprotein complexes, scaffolding networks, and signaling pathways that allow cells to sense and respond to changes in their environment.
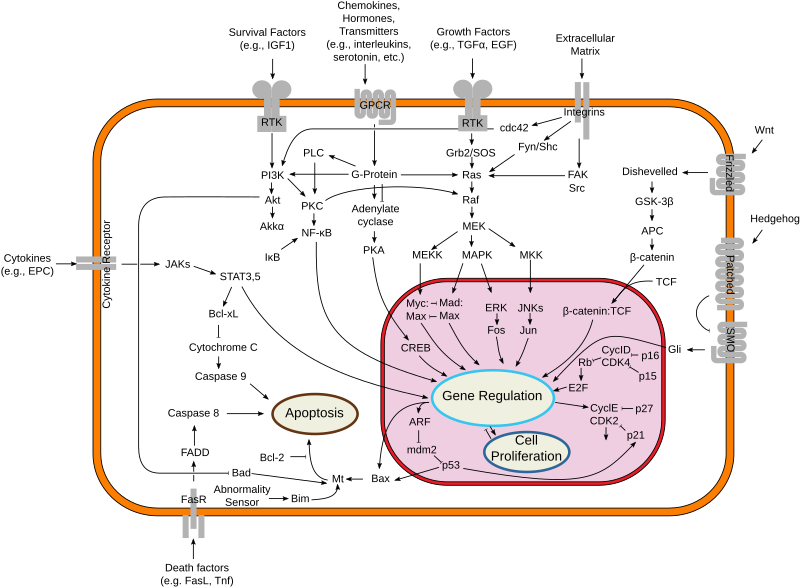
A. Signal Transduction
Many signaling pathways rely on PPIs to propagate signals from the cell surface to the nucleus. These interactions allow for the rapid and coordinated activation of downstream effectors.
- Example: The Ras-Raf-MEK-ERK pathway is a classical example of a signaling cascade that depends on PPIs. The activation of Ras by a membrane-bound receptor leads to the recruitment of Raf, which then phosphorylates and activates MEK and ERK through transient PPIs. ERK translocates to the nucleus to activate transcription factors involved in cell proliferation.
B. Enzyme Complexes
PPIs are critical for the formation of multi-enzyme complexes that carry out sequential biochemical reactions, enhancing the efficiency and specificity of metabolic pathways.
- Example: In the citric acid cycle, enzymes like citrate synthase and aconitase interact with one another to catalyze successive steps of the cycle. These interactions ensure that intermediates are efficiently passed from one enzyme to the next.
C. Structural Maintenance
PPIs also play essential roles in maintaining the structural integrity of cells and tissues. Cytoskeletal proteins such as actin and tubulin polymerize through homomeric interactions to form filaments that provide mechanical support.
- Example: Microtubules, formed by the heteromeric interaction of α- and β-tubulin, are involved in cell division, intracellular transport, and the maintenance of cell shape.
5. Studying Protein-Protein Interactions: Experimental Methods
Several experimental techniques have been developed to study PPIs, each with unique advantages and limitations. These methods allow researchers to identify interaction partners, measure binding affinities, and understand the structural basis of protein complexes.
A. Co-Immunoprecipitation (Co-IP)
Co-IP is a widely used technique for detecting PPIs in living cells. It involves using an antibody specific to one protein of interest to "pull down" the entire protein complex, allowing identification of associated proteins by mass spectrometry or Western blotting.
- Limitations: Co-IP often requires high-affinity interactions and may miss transient or weak interactions.
B. Yeast Two-Hybrid (Y2H) System
The yeast two-hybrid system is a genetic technique used to detect binary PPIs. It involves the reconstitution of a functional transcription factor when two proteins of interest interact, leading to the activation of a reporter gene.
- Limitations: The Y2H system is limited to interactions that occur in the yeast nucleus and may miss interactions that require post-translational modifications or specific cellular conditions.
C. Surface Plasmon Resonance (SPR)
SPR is a biophysical technique used to measure the kinetics and affinities of PPIs. It involves immobilizing one protein on a sensor chip and flowing the interacting partner over the surface, allowing real-time monitoring of the interaction.
- Advantages: SPR provides detailed information about binding kinetics, including association and dissociation rates.
D. X-ray Crystallography and Cryo-EM
To understand the structural basis of PPIs, high-resolution techniques such as X-ray crystallography and cryo-electron microscopy (cryo-EM) are used to determine the three-dimensional structures of protein complexes.
- Example: Cryo-EM has recently been used to elucidate the structure of large protein complexes, such as the spliceosome, revealing the intricate network of PPIs that govern mRNA splicing.
6. Disruption of Protein-Protein Interactions in Disease
Disruptions in PPIs are often implicated in diseases such as cancer, neurodegenerative disorders, and infectious diseases. Mutations that alter the binding interface of proteins can lead to the loss of critical interactions, while aberrant interactions can result in toxic protein aggregates or hyperactivation of signaling pathways.
- Example: In cancer, mutations in the tumor suppressor protein p53 often disrupt its interaction with other regulatory proteins, impairing its ability to control cell proliferation and apoptosis. As a result, the loss of functional PPIs involving p53 leads to uncontrolled cell growth and tumor development.
Protein-protein interactions are the foundation of cellular machinery, orchestrating virtually every biological process. From signaling to structural maintenance, the dynamic and complex nature of these interactions allows cells to function efficiently and adapt to their environment. Understanding PPIs at a molecular level is not only fundamental to cell biology but also has profound implications for disease research and drug discovery. Through advances in proteomics and structural biology, we continue to uncover the intricate web of protein interactions that underpins life.
Recent Posts
-
Can mNGS Replace Culture?
In microbiology and infectious-disease work, culture has been the gold standard for over a century. …30th Sep 2025 -
Post-Translational Modifications: The Hidden Layers of Protein Regulation
Proteins are the workhorses of cellular biology, performing a wide array of functions that are essen …18th Oct 2024 -
Unveiling the Structure-Function Relationship in Proteins: Why Shape Matters
Proteins are fundamental biomolecules that drive virtually every biological process within an organi …18th Oct 2024